The EuroPainClinics® Study I (EPCS I) study is focused on clinical research in the treatment method of periradicular therapy (PRT).
The EuroPainClinics® Study I (EPCS I) study is focused on clinical research in the treatment method of periradicular therapy (PRT) as a treatment of root syndromes caused by nerve root compression due to various pathological processes that cause the so-called radicular pain. These are most often patients who have been diagnosed with protrusion, arching or prolapse of the intervertebral disc on the basis of a CT or MR examination. The study aims to monitor long-term pain relief and improve the quality of life of patients undergoing PRT.
On April 23, 2015, the study was approved by the Regional Hospital Ethics Committee under EC number: 74 / EK / 15. It was subsequently registered in the international database of the National Health Service USA https://clinicaltrials.gov PRS: NCT02464553. EPCS 1 Approval document
The project meets all the attributes and valid legislative standards related to medical procedures approved by the Ministries of Health in the Slovak Republic and the Czech Republic.
Study detail
The EuroPainClinics®Study I (EPCS I) study is focused on clinical research of the treatment method of periradicular therapy (PRT)
Periradicular therapy (PRT) – indication criteria
This therapy is aimed at the treatment of root syndromes caused by nerve root compression by various pathological processes. PRT is one of the treatment events in which anti-inflammatory drugs are injected near the nerve root together with a local anesthetic.
Radicular pain (pain caused by irritation of the spinal nerve roots) is most often caused by compression of the roots in the spinal canal. Compression can be caused by various conditions such as foraminal stenosis, spinal canal stenosis or postoperative fibrosis in the epidural space.
It should be kept in mind that PRT cannot be performed for every condition causing spinal cord compression. An example of a contraindication is a released sequester – fragment, malignant disease, severe acute or chronic inflammatory disease, coagulopathy, etc.
Subject of the EPCS I study and registration
The EPCS I study aims to monitor long-term pain relief and improve the quality of life in patients who undergo PRT and who meet the indicative inclusion criteria below.
The subject of the project is the implementation of a multicenter double-blind randomized clinical study dealing with the comparison of two methodological procedures for performing periradicular therapy. The study was prepared and prepared by Europainclinics z.ú. After elaboration of the study design, preparation of informed consents for study participants and study protocol, the study was subsequently approved on April 23, 2015 by the regional hospital ethics committee under the EC number: 74 / EK / 15. Subsequently, it was registered in the international database National health service U.S
https://clinicaltrials.gov PRS: NCT02464553. The project meets all attributes and valid legislative standards regarding the medical output approved by the Ministries of Health in the Slovak Republic and the Czech Republic. The processing of personal data for patients in order to conduct a study is in line with the current version of the 2008 Helsinki Declaration and in accordance with the country’s relevant laws. Patient personal data protection will be maintained. Data will be based on EU directives: Directive 95/46 / EC, Directive 2002/58 / EC, Directive 2006/24 / EC. It will ensure that the study protocol, informed consents with the procedure and inclusion in the study are according to the local requirements provided by the relevant independent ethical committee. In the event that the laws of the country require this, EuropainClinics is responsible for providing an annual update of the documentation to an independent ethical committee. The results obtained in the study will be published in professional international journals and considered in the recommendations and procedures in the clinical practice of interventional pain management.
EPCS I study
The study compares two groups of patients with pathological findings in the lumbosacral area where periradicular therapy is indicated. The inclusion criteria include MR findings of spinal cord compression, while the level of clinical symptomatology responds to the dermatome one level below the lesion.
The patient fulfilling the inclusion criteria will be informed by the treating physician about the option to take part in the study and will be offered to participate. In the case of patient consent, they will be instructed about the PRT intervention and will subsequently sign an informed consent to participate in the study and an informed consent about the invasive procedure – PRT, in the levels of one or two intervertebral spaces. The patient will not know which of these two procedures they will undergo. The patient agrees that detailed information about the procedure will be disclosed after the end of the study. However, the patient may at any time request a detailed report on the procedure performed. In this case, all data relating to the previous therapeutic process will be provided immediately. At the same time, they will be excluded from the monitored set of study patients. The patient will be assigned an identification number generated by a random number generator. Upon completion of the first protocol from a clinical examination prior to the procedure, the protocol will be sent to the study coordinator and the person in charge of the study data processing. The first and second postoperative patient examination will be carried out by another physician (not performing the procedure itself), in another interventional pain management workplace and fill in additional clinical examination reports.
Patients meeting the inclusion criteria will be randomly divided into groups A and B. Patients classified into a group A1 undergo a minimally invasive procedure during which PRT will be performed in one intervertebral space. Patients included in Group B1 will undergo a minimally invasive procedure during which PRT will be performed in two adjacent intervertebral spaces.
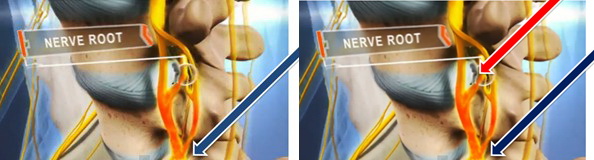
- Group A1 patients: injection at the site corresponding to symptomatology
- Group B 2: injection at the site corresponding to symptomatology and in the area of MRI verified nerve root compression
The first post-operative follow-up examination is organized by the study coordinator after 6 months and the second postoperative follow-up examination after 12 months.
EPCS I Objectives I and Tracked Parameters
The aim of the project is a comparison of the clinical condition of patients who have undergone PRT performance after fulfilling the inclusion criteria to take part in the EPCS I study. From the monitored parameters, clinical status, visual scale of pain, global pain score, pain dissemination in the respective dermatomes, reduction in painkiller medication consumption, OSWESTRY Disability Questionnaire (assessing the quality of life in patients with lumbosacral pain) will be compared. The monitored parameters will be recorded in three time periods: prior to the procedure (first examination), subsequently 6 months after the procedure (second examination) and finally 12 months after the procedure (third examination). The results will subsequently be subjected to statistical analysis. Another objective of the project is to disclose the obtained results in professional international journals.
